ARRIVE study plan
The ARRIVE study plan is a tool for researchers, technicians and ethical review bodies, offering a structured template to promote transparent communication of planned in vivo studies.
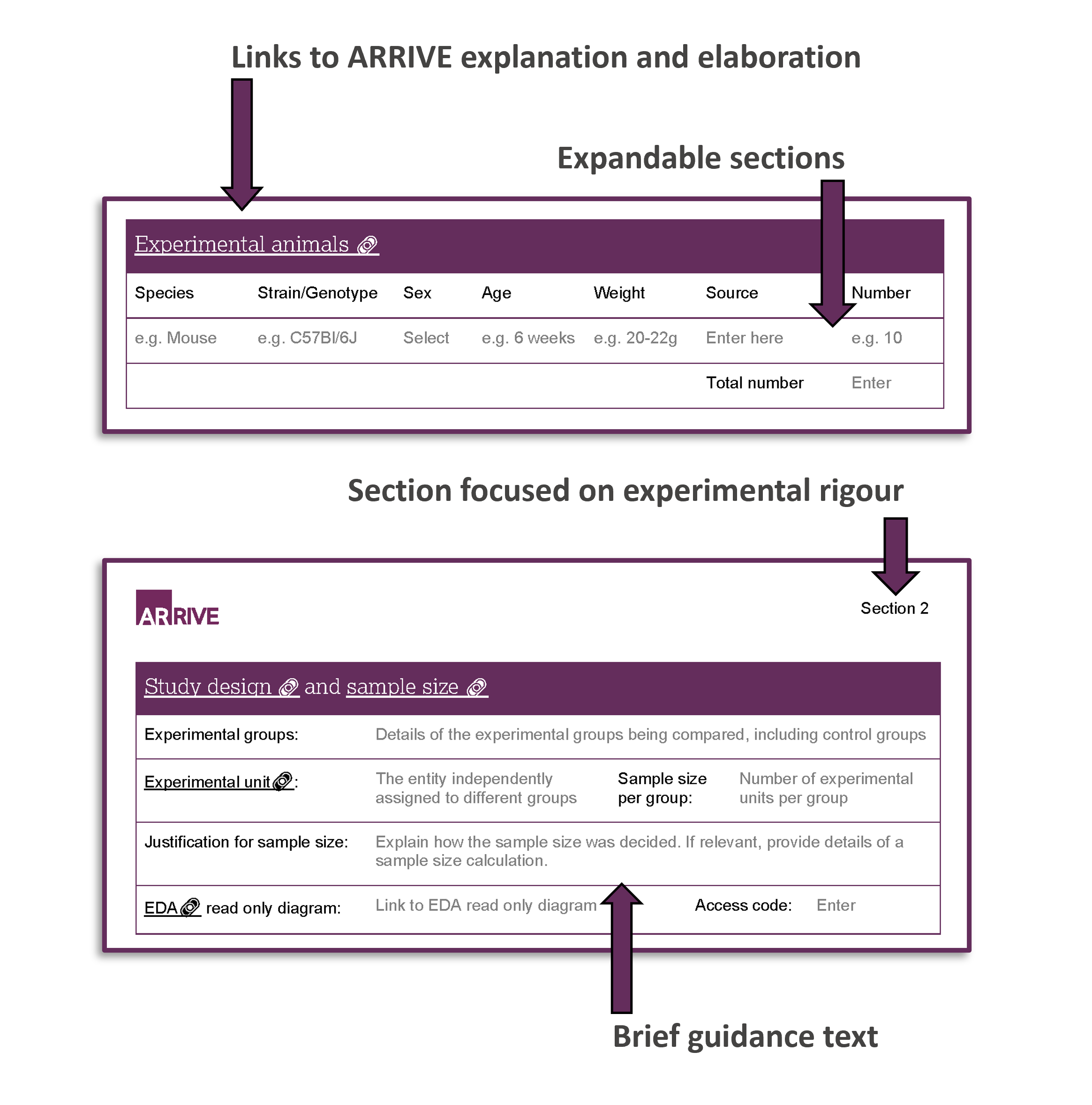
Designed to support good experimental practices from the outset, the ARRIVE study plan ensures that each animal research experiment is considered carefully. By documenting the procedural plans and experimental design (e.g. minimising bias, selecting appropriate sample sizes and employing suitable statistical analyses) in a clear and standardised format, the template facilitates a thorough review of a prospective study by animal care staff and ethical review committees. This enables an assessment of the rigour of the research, the refinement of the procedures and the likelihood of discovering reliable findings.
The ARRIVE study plan fosters collaboration between researchers and animal units by clearly communicating the planned procedures and rigour strategies to be used. This approach sets a consistent practice that all parties can follow, helps to ensure compliance with relevant training and project records, and contributes to the successful conduct of in vivo research. A webinar recording is available below to understand more about use of study plans in academic institutions.
We strongly recommend using the NC3Rs Experimental Design Assistant (EDA) alongside the ARRIVE study plan. The EDA offers tailored advice on study design and analysis, and its workflow diagram can be integrated into the study plan, providing a visual representation of the planned in vivo experiment. This integration further enhances transparency and aids in the effective communication of the study's design.
Before embarking on research involving the use of animals it is also critical to form a clear hypothesis, identify possible non-animal alternatives to all or part of the proposed study and assess the relevance of the chosen model to answer the experimental question. We encourage researchers to consult the PREPARE guidelines before considering the use of animals in research. PREPARE provides researchers with an extensive overview to formulating an experiment and the requirements of using animals before carrying out the research.
Why use the ARRIVE study plan?
For technicians and animal units
-
Provides documentation for record keeping and monitoring compliance.
-
Includes all procedural and welfare details.
-
Identify opportunities to support rigorous science.
For researchers
-
Links and guidance on experimental design support.
-
Promotes the use of rigorous, unbiased experimental methods.
-
Provides a record for full and transparent reporting of in vivo studies.
For ethical review bodies
-
Maximises the use of existing infrastructure for greater 3Rs impact.
-
Assists in the harm-benefit analysis during ethical review.
-
Promotes best practice in the conduct of animal research.
If you require an accessible format of the ARRIVE study plan, please email arrive@nc3rs.org.uk.
Other resources
This webinar explores the development, benefits and features of the ARRIVE study plan.
In this webinar, Lauren Cresser, HOLC and NTCO, explains how The Pirbright Institute uses study plans to maximise the benefits of their research.

The NC3Rs developed the Experimental Design Assistant (EDA), a free-to-use online tool, to help design robust experiments more likely to yield reliable and reproducible results.
The EDA helps you build a diagram representing your experimental plan, which can be critiqued by the system to provide bespoke experimental design feedback. You can then produce a URL to a read-only EDA diagram to include in an ARRIVE study plan.
